Answer:
Option D.
Explanation:
Density of Hydrogen =
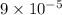
Density of Helium =
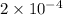
Now ratio of the densities =
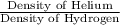
=
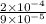
= 0.22×
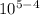
= 2.2
Density of Helium = 2× Density of Hydrogen
Therefore, density of Helium is approximately 2 times the density of Hydrogen.
Option D is the answer.