Answer: 3M
Step-by-step explanation: Molarity : It is defined as the number of moles of solute present in one liter of solution.
Formula used :
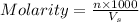
where,
n = moles of solute
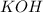 = 0.6 moles
= 0.6 moles
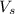 = volume of solution in ml= 200 ml
= volume of solution in ml= 200 ml
Now put all the given values in the formula of molarity, we get
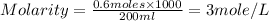
Therefore, the molarity of solution will be 3M.