Answer:
a) 5.40 g B :
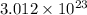 atoms
atoms
b) 0.250 mol K :
 atoms
atoms
c) 0.0384 mol K :
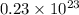 atoms
atoms
d) 0.02550 g Pt:
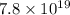 atoms
atoms
e)
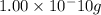 Au:
Au:
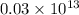 atoms
atoms
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
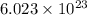 of particles.
of particles.
To calculate the moles, we use the equation:
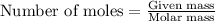
a) 5.40 g B
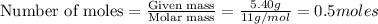
1 mole of boron contains =
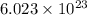 atoms
atoms
Thus 0.5 moles of boron contain =
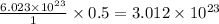 atoms
atoms
b) 0.250 mol K
1 mole of potassium (K) contains =
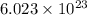 atoms
atoms
Thus 0.250 moles of potassium contain =
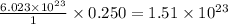 atoms
atoms
c) 0.0384 mol K
1 mole of potassium (K) contains =
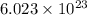 atoms
atoms
Thus 0.0384 moles of potassium (K) contain =
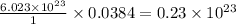 atoms
atoms
d) 0.02550 g Pt
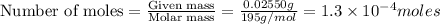
1 mole of platinum contains =
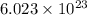 atoms
atoms
Thus
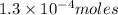 of platinum contain=
of platinum contain=
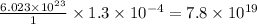 atoms
atoms
e)
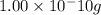 Au,
Au,
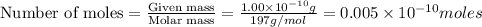
1 mole of gold contains =
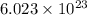 atoms
atoms
Thus
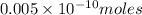 of platinum contain=
of platinum contain=
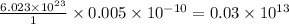 atoms
atoms