Answer : - 136.0 KJ/mol
Explanation : The complete question is attached below; assuming that the bond energies are given as per the question to find out the heat of formation of
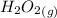 we need to draw its lewis dot structure for this reaction;
we need to draw its lewis dot structure for this reaction;
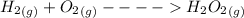
 will have H-H bond and
will have H-H bond and
 will have O=O with 2 pairs of lone electrons on each of O atom.....These are the bonds which are broken
will have O=O with 2 pairs of lone electrons on each of O atom.....These are the bonds which are broken
In
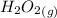 has H-O-O-H bonds; Here, O atom again has 2 pairs of lone pairs on each O atom; ....These are bonds which are made
has H-O-O-H bonds; Here, O atom again has 2 pairs of lone pairs on each O atom; ....These are bonds which are made
ΔH°f = ∑ {ΔH(bonds broken) - ΔH (bonds made)}
ΔH°f = ∑ { (432 + 494) - [(459 X 2) +142] }
On solving, We get,
ΔH°f = -136.0 KJ/mol
Hence, the heat of formation for
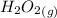 will be -136.0 KJ/mol
will be -136.0 KJ/mol