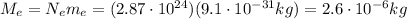Let's calculate the total charge of M=4.8 g=0.0048 kg of protons.
Each proton has a charge of
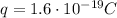
, and a mass of
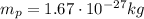
. So, the number of protons is
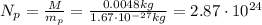
And so the total charge of these protons is
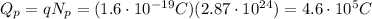
So, the neutralize this charge, we must have
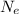
electrons such that their total charge is
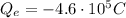
Since the charge of each electron is
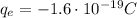
, the number of electrons needed is
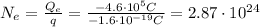
which is the same as the number of protons (because proton and electron have same charge magnitude). Since the mass of a single electron is
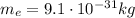
, the total mass of electrons should be