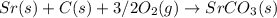Answer:
Step-by-step explanation:
The standard enthalpy of formation (ΔH°f) is defined as the enthalpy change which accompanies the formation of 1 mole of a compound from its constituent elements all of which are in their standard states i.e. either solid (s), liquid (l) , gas (g) or the aqueous phase (aq).
Strontium carbonate (SrCO3) contains 3 elements:
-Strontium (Sr) where the standard state is solid(s)
- Carbon (C) where the standard state is again a solid (s)
- oxygen (O) where the standard state is the gas phase (g)
The balanced chemical equation for the standard enthalpy of formation (ΔH°f) for SrCO3 would be: