Step-by-step explanation:
A balanced chemical reaction is a reaction in which there is equal number of atoms on both reactant and product side. Also, the mass of atoms or compounds present on reactant side equals the mass of atoms or compounds present on product side.
For example,
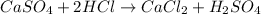
Molar mass of
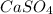 = 136.14 g/mol
= 136.14 g/mol
Molar mass of 2
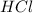 =
=
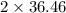 g/mol = 72.92 g/mol
g/mol = 72.92 g/mol
Therefore, sum of molar mass of reactants = (136.14 + 72.92) g/mol
= 209.05 g/mol
Molar mass of
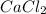 = 110.98 g/mol
= 110.98 g/mol
Molar mass of
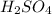 = 98.07 g/mol
= 98.07 g/mol
Therefore, sum of molar mass of products = (110.98 + 98.07) g/mol
= 209.05
Therefore, we can see that it is true that when you balance a chemical reaction, you are making sure that the law of conservation of matter is obeyed.