Answer:
0.10 mol of sulfuric acid will neutralize 0.10 mol of calcium hydroxide.
Step-by-step explanation:
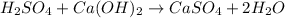
1 mol of sulfuric acid reacts or neutralizes with 1 mol of calcium hydroxide.
Then 0.10 mole of calcium hydroxide will be neutralized by:
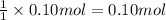 of sulfuric acid
of sulfuric acid
0.10 mol of sulfuric acid will neutralize 0.10 mol of calcium hydroxide.