Slower to fastest
Kr, NO₂,N₂, CH₄
Further explanation
Given
Molecules :
Nitrogen dioxide, Methane, Nitrogen, Krypton
Required
Average molecular speed.
Solution
Average velocities of gases can be expressed as root-mean-square averages. (V rms)
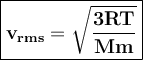
R = gas constant, T = temperature, Mm = molar mass of the gas particles
So average molecular speed inversely proportional to the molar mass
The larger the molar mass the slower the gas velocity
Molar mass NO₂ = 46 g/mol
Molar mass CH₄ = 16 g/mol
Molar mass N₂ = 28 g/mol
Molar mass Kr = 84 g/mol
slower to fastest :
Kr, NO₂,N₂, CH₄