Answer:
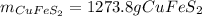
Step-by-step explanation:
Hello,
In this case, the set of chemical reactions are shown below:
(1) 2 CuFeS₂ + 3 O₂ ----> 2 CuS + 2 FeO + 2 SO₂
(2) 2 FeO + SiO₂ ----> 2 FeSiO₃
(3) 2 CuS ----> Cu₂S + S
(4) Cu₂S + S + O₂ ----> 2 Cu + 2 SO₂
In such a way, the pennies are assumed to be 100% copper, and each penny has about 3.0 g of copper, so based on the 4th reaction, we compute moles of Cu₂S and pass through until the 1st reaction as shown below:
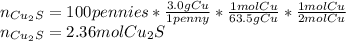
Now, from the 3rd reaction we compute the moles of CuS:
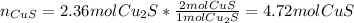
2nd reaction is needless, so we proceed to compute CuS's theoretical amount since the 4.72 mol of CuS are said to be actually obtained (real amount) as shown below:
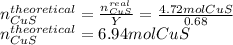
Now, we develop the shown-below stoichiometric relationship between CuS and the chalcopyrite to compute the required amount to be mined in grams, for example
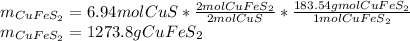
Best regards.