Answer:
There is
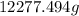 of CO2
of CO2
Step-by-step explanation:
We know that the molar mass of CO2 is
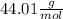
This means that in 1 mole of molecules of CO2 is 44.01 g of CO2
In one mole of molecules of any substance there are N molecules where N is defined as the Avogadro number.
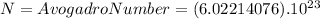
Therefore, we can write that
In
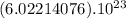 molecules of CO2 (1 mole of CO2) there is 44.01 g of CO2 ⇒
molecules of CO2 (1 mole of CO2) there is 44.01 g of CO2 ⇒
In
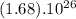 molecules of CO2 there will be x grams :
molecules of CO2 there will be x grams :
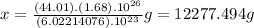
We answer that in
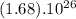 molecules of CO2 there is
molecules of CO2 there is
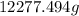 of CO2
of CO2