Hello!
The reaction for the dissolving of solid copper with HNO₃ is the following:
Cu (s) + 4HNO₃ (aq) → Cu(NO₃)₂ (aq) + 2NO₂ (g) + 2H₂O (l)
The required mL to neutralize 0,18 g of solid copper are calculated using the following conversion factor:
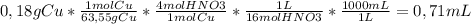
Now we subtract this value to the volume of HNO3 added by the student:
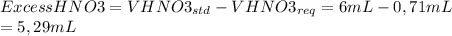
To finish, we calculate the volume of NaOH 6M required to neutralize this amount of 16M acid in excess. The reaction is the following:
HNO₃ + NaOH → NaNO₃ + H₂O
To calculate this value, we use the following conversion factor:
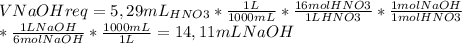 So, you'll need 14,11 mL of 6M NaOH to neutralize the excess acid
So, you'll need 14,11 mL of 6M NaOH to neutralize the excess acid