Answer: 103.9082 grams of HCl will be produced by 135 grams of titanium tetra chloride.
Step-by-step explanation:
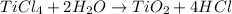
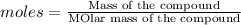
Number of moles of
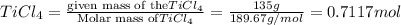
According to reaction, 1 mole
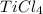 produces 4 moles of HCl , then 0.7117 moles of
produces 4 moles of HCl , then 0.7117 moles of
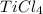 will produce :
will produce :
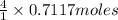 of HCl that is 2.8468 moles.
of HCl that is 2.8468 moles.
Mass of HCl =
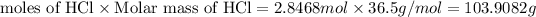
103.9082 grams of HCl will be produced by 135 grams of titanium tetra chloride.