Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, we can proceed as follows:
1. Here, the undergoing chemical reaction is:
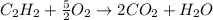
Thus, the moles and mass of water turn out:
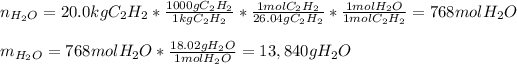
2. Here, the undergoing chemical reaction is:
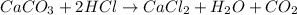
So the required moles of HCl and the yielded of water are:
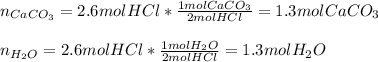
3. Here, the undergoing chemical reaction is:
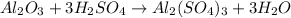
Now, we apply each mole ratio obtain:
A.
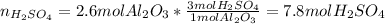
B.
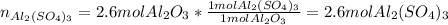
Best regards!