Answer:
A) solution 2 is more acidic than solution 1
B) Graph in attachment
C) Ratio method prefer
Explanation:
A chemist prepares two acid solutions.
Solution 1: 3 parts sulfuric acid to 7 parts water
Ratio of sulphur to water
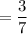
Solution 2: 4 parts sulfuric acid to 9 parts water
Ratio of sulphur to water
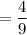
Part A: Using ratio to find the more acidic
First we make same denominator
Solution 1:
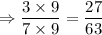
Solution 2:
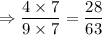
As 28 > 27
Therefore, solution 2 is more acidic than solution 1
Part B: Let y amount of sulfuric acid mix with x amount of water.
Equation of solution 1:
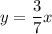
Equation of solution 2:
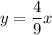
Please find attachment for graph.
Part C: part A method is more prefer to find more acidic. Because it gives actual amount of acidic.