Step-by-step explanation:
A solid state is represented by (s) in a chemical reaction equation whereas when a compound is soluble in the solution then it is said to be aqueous solution and it is denoted as (aq) in a chemical reaction equation.
On the other hand, gas is denoted by a symbol (g) in the chemical reaction equation.
Therefore, in the reaction
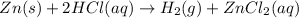 , zinc chloride (
, zinc chloride (
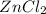 ) product is a substance that is dissolved in solution.
) product is a substance that is dissolved in solution.