Answer:
W =
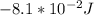
Step-by-step explanation:
The rest of the question actually states that:
**If the average net pressure is 1.5x10^3Pa and the balloons volume increases by 5.4x10^-5 m^3, how much work is done by the expanding gas.**
Work done, W against an external pressure,
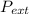 , which causes a change in volume, ΔV of an object is given as:
, which causes a change in volume, ΔV of an object is given as:
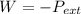 ΔV
ΔV
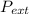 =
=
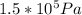
ΔV =
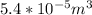
=>
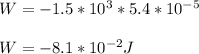
Note: The negative sign denotes that the work is done against external pressure.