Answer : Option C) Atomic Size
Explanation : The atomic radius of the elements is found to be decreasing if we go from left to right in the modern periodic table. Accordingly,
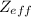 increases as the number of shielding electrons present in the atomic nucleus of the periodic elements which lies in the same row remains constant while the number of protons in each atomic shell increases.
increases as the number of shielding electrons present in the atomic nucleus of the periodic elements which lies in the same row remains constant while the number of protons in each atomic shell increases.
The effective nuclear charge
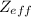 of an atom is defined as the net positive charge which is felt by the valence electron of the atomic element.
of an atom is defined as the net positive charge which is felt by the valence electron of the atomic element.
When
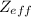 is observed to decrease, it is seen that the atomic radius grows in size. So, it explains the inverse relationship between both. This phenomenon occurs, because there is more screening of the electrons from the nucleus taking place, which is observed due to decrease the attraction between the electron and the nucleus.
is observed to decrease, it is seen that the atomic radius grows in size. So, it explains the inverse relationship between both. This phenomenon occurs, because there is more screening of the electrons from the nucleus taking place, which is observed due to decrease the attraction between the electron and the nucleus.