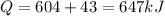Answer:
Heat transferred to the gas is given as
Q = 647 kJ
Step-by-step explanation:
As per first law of thermodynamics we know that
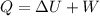
now here we know that
change in internal energy of the gas is
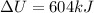
Also the steam expands so we will have
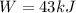
since volume increases to here work is done by the gas
now from above equation the heat given to the system is