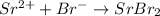Step-by-step explanation:
Strontium is a metal whereas bromine is a non-metal. Therefore, strontium will donate its excess electrons to bromine atom in order to become stable. Hence, both Sr and Br will combine together to form an ionic bond.
The reaction between strontium and bromine is as follows.