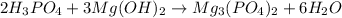Answer 1: The correct answer is option b.
Explanation: At room temperature that 25 °C water is present as in its liquid state. Water exists as solid on 0 °C and below and as gas on 100 °C and above.
Answer 2: The correct answer is option c.
Explanation:An ion is formed when an atom either gains electrons(anion) or loose electrons(cation).
Answer 3:The correct answer is option c.
Explanation: Gas is one of the state of matter which has no definite shape and size.It takes the shape and size of the container in which it is kept. But gas have definite volume.
Answer 4: The correct answer is option d
Explanation: Sublimation is defined as the process of the conversion of matter from solid state to gaseous state directly by escaping liquid state.
Answer 5: The correct answer is option b.
Explanation: Matter is defined as anything which occupies some space and has mass.
Answer 6:The correct answer is option c
Explanation: The particle with positive charge is called proton. Where as particle with negative charge is called electrons and particle with no charge is called neutron.
Answer 7: The correct answer is option d.
Explanation: Mass number = Number of protons + Number of neutrons
24 = 11 + number of neutrons
Number of neutrons = 12
Answer 8: The correct answer is option c.
Explanation: Electron are not stationary they are all time moving. so, when the electrons moving in their lowest energy configuration they are said to be ground state
Answer 9: The correct answer is option c.
Explanation: Synthesis reaction is defined as those chemical reactions in which two or more than two reactants reacts together to form single product.
A+B+C →D
Answer 10: The correct answer is option c.
Step-by-step explanation:
Dalton's Atomic Theory
- An atom is an indivisible and smallest unit of matter.
- Atom of same element have similar mass and size.
- Atoms of different elements differ in size and mass
- Chemical reaction means change in arrangement of atoms.
Answer 11: The correct answer is option c.
Explanation: Electron near the nucleus experiences stronger electrostatic forces due to which they posses significantly higher energy than electrons further away.
Answer 12: The correct answer is option 2.
Explanation: The maximum electron capacity of s orbital is 2.
Where as for p orbital it is 6, for d orbital it is 10 and for f orbital it is 14.
Answer 13: The correct answer is option d.
Explanation: An atom retains all the properties of its respective element.
Answer 14: The correct answer is option a.
Explanation: Symbol of copper is Cu.
C is for carbon, Ca is for calcium and Pb is for lead.
Answer 15: The correct answer is option d.
Explanation: Horizontal rows are called period and vertical columns are called group.
Answer 16: The correct answer is option b.
Explanation:
Metals are good conductor of electricity and heat due to presence of metallic bonding . Where as in non metal covalent bonding is present.
Answer 17: The correct answer is option a.
Explanation: Electronegativity is defined as the ability to attract the shared pair of an electrons towards itself in bond.
Fluorine being smallest in size and valence electrons are closer to nucleus due to which gain of an electron becomes easier.
Answer 18: The correct answer is option b.
Explanation: Ionic bond is the chemical bond in which complete transfer of electron takes place from one atom to another
Answer 19: The correct answer is option d.
Step-by-step explanation:
An electrolyte those compound which when dissolved in water dissociates into ions and make the solution electrical conducting in nature.
Answer 20: The correct answer is option b.
Step-by-step explanation:
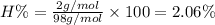
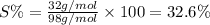
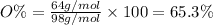
Answer 21: The correct answer is option a.
Step-by-step explanation:
Moles of sodium =
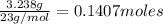
Moles of Sulfur=
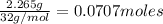
Moles of oxygen =
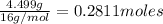
Divide number of moles of every element with smallest value of number of moles.
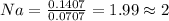
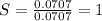
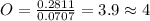
The empirical formula is
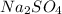
Answer 22: The correct answer is option c.
Step-by-step explanation:
Combustion reaction is the burning of organic substance in presence of oxygen gas with release of water , carbon-dioxide ans heat.
Answer 23: The correct answer is option a.
Step-by-step explanation:

Sodium bromide NaBr.
Answer 24: The correct answer is option c.
Step-by-step explanation: