Answer: Decrease the temperature by 25°C and decrease the pressure by 0.500 atm.
Explanation:
STP stands for standard temperature and pressure conditions.
STP is defined as a temperature of
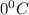 and an absolute pressure of exactly 1 atm.
and an absolute pressure of exactly 1 atm.
Thus in order for a gas sealed in bottle at
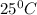 and 1.50 atmosphere to bring to STP , the temperature must be decreased by
and 1.50 atmosphere to bring to STP , the temperature must be decreased by
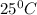 and pressure must be decreased by 0.5 atm to make it
and pressure must be decreased by 0.5 atm to make it
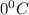 and 1 atmosphere respectively.
and 1 atmosphere respectively.