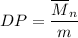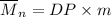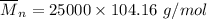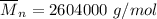Answer:
Step-by-step explanation:
In Polystrene, the molecular formula for the repeat unit =
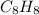 ;
;
and the atomic weights of Carbon C = 12.01 g/mol
For Hydrogen, it is 1.01 g/mol
Hence, the repeat unit molecular weight is:
m = 8 (12.01 g/mol)+8(1.01 g/mol)
m = 96.08 g/mol + 8.08 g/mol
m = 104.16 g/mol
The degree of polymerization = no-average molecular weight/repeat unit molecular weight.
Mathematically;