Step-by-step explanation:
According to Avogadro's law, the pressure of the gas is directly proportional to the number of gas molecules. This means when the temperature and the volume of the system remains constant, as the number of gas molecules increases the pressure will also increase.
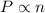
n is the number of gas molecules.
Hence, it is clear that there exists a direct relationship between the pressure and the number of gas molecules.