Answer: The reactant pair Na + BaO will not react to produce a reaction.
Explanation: To study, if the reaction occurs or not, we should know about the reactivity series of the metals.
The metal which lies above in the reactivity series will easily displace the metals which lie below in the reactivity series.
The reactions which is based on the reactivity series are known as displacement reactions.
The reactants given in the question undergo single displacement reaction.
Single displacement reactions are defined as the reactions in which a more reactive element displaces the least reactive metal in a given chemical reaction.
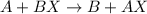
were, A is more reactive than B.
From the given reactant pairs, only one will not undergo a chemical reaction.
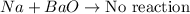
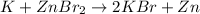
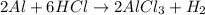
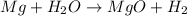
The reactant pair (Na + Ba) will not undergo a reaction.