Answer:
The volume of Neon would be 15290811,94 pm^3
Step-by-step explanation:
In order to solve this we just have to calculate the volume that the neon will occupy, its radius is 0,69 A or 154 pm with this we just have to calculate with the next formula:
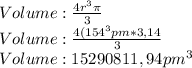
So the volume of Neon would be 15290811,94 pm^3