Answer : The correct options are, (C), (D) and (F)
Explanation :
Redox reaction : It is also know as an oxidation-reduction reaction in which the electrons transferred between the two species. In this, one species shows oxidation by losing electrons and another species shows reduction by gaining electrons.
Most of the chemical reactions shows the redox reaction. The chemical reactions like, synthesis, combustion, single replacement, decomposition shows the redox reaction.
As, the reactants of Option A, B and E does not shows redox reaction because in these reactions the metals will not displace the another metal ion due to less reactivity of metal.
While the reactants of option C, D and F shows redox reaction because in these reactions the metals will displace the another metal ion easily due to high reactivity of metal.
The reactivity series is shown below.
The balanced redox reactions will be,
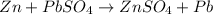
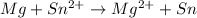
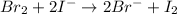
Hence, the correct options are, (C), (D) and (F)