Final answer:
Aluminum oxide is an ionic compound, not a covalent compound, with the formula Al₂O₃.
Step-by-step explanation:
False. Aluminum oxide is not a covalent compound; it is an ionic compound. Ionic compounds typically form between metals and nonmetals, where electrons are transferred from the metal to the nonmetal.
Aluminum oxide
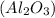 is formed by the reaction of aluminum, a metal, with oxygen, a nonmetal:
is formed by the reaction of aluminum, a metal, with oxygen, a nonmetal:
![\[4Al + 3O_2 \rightarrow 2Al_2O_3\]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vnshfwi4k0kuymcxji1hognp31pb06l77x.png)
In this reaction, aluminum (Al) loses electrons to form
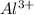 ions, and oxygen
ions, and oxygen
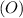 gains electrons to form
gains electrons to form
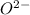 ions. The resulting compound, aluminum oxide, has a three-dimensional array of ions held together by ionic bonds. This is in contrast to covalent compounds, where electrons are shared between atoms.
ions. The resulting compound, aluminum oxide, has a three-dimensional array of ions held together by ionic bonds. This is in contrast to covalent compounds, where electrons are shared between atoms.
In summary, aluminum oxide is an ionic compound due to the transfer of electrons between aluminum and oxygen atoms.