Answer 1)
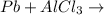 no reaction
no reaction
As lead lies lower in reactivity series, it is less reactive than Aluminium. It will not be able to displace aluminium from its salt and hence no reaction occur. Thus the given statement is false.
2)
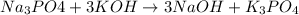 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction.
A balanced chemical reaction is one in which the number of atoms on both sides of a chemical equation are same. Also K has an oxidation state or charge of +1 in
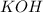 as well as
as well as
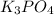
3)
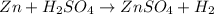
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.Thus zinc can easily lose electrons as compared to hydrogen and result in the formation of zinc sulfate and hydrogen.
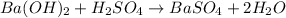 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place.
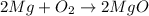 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.
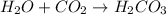 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.
4)
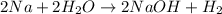 Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium easily lose electrons than hydrogen and get oxidized to
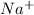 in NaOH and
in NaOH and
 get reduced to give
get reduced to give

5) A single replacement reaction is a reaction in which one element replaces a similar element within a compound - False
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution. Thus one element should be different from another element.
A double displacement reaction is one in which exchange of ions take place.
6) Metal + Ionic compound : It is a single replacement reaction, and the cations in the two ionic compounds are different.
Example:
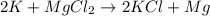 where K is a metal and
where K is a metal and
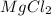 is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.
is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.