Answer:
184.3g
Step-by-step explanation:
Given parameters:
Volume of acid = 42.5L
Unknown:
Mass of acid = ?
Solution:
We have to assume that this measurement was taken at rtp;
At rtp;
1 mole of a substance occupies a volume of 22.4L
Therefore,
42.5L of H₂SO₄ will have
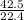 moles = 1.9moles
moles = 1.9moles
To find the mass;
Mass = number of moles x molar mass
Molar mass of H₂SO₄ = 2(1) + 32 + 4(16) = 97g/mol
Mass = 1.9 x 97 = 184.3g