Answer:
A. 8.47 * 10^-6
Step-by-step explanation:
Avogadro's number is used to convert moles to molecules, but it can also be used to convert moles into moles. Avogadro's number is 6.022 * 10^23 molecules per mole (or atom).
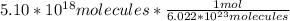
The molecules units cancel leaving us with:
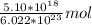
This equals approximately 8.47 * 10^-6, which is the first option, A.
Hopefully this helped. Good luck!