The question is incomplete. Here is the complete question.
Aqueous solutions of
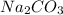 and
and
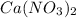 , 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?
, 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?
Observation upon Observation upon
addition of precipitate addition of precipitate
to NaCl(aq) to HCl(aq)
(A) additional precipitates forms no visible reaction occurs
(B) no visible reaction occurs gas is produced and some precipitate dissolves
(C) no visible reaction occurs no visible reaction occurs
(D) additional precipitates forms gas is produced and some
precipitate dissolves
Answer: (B) No visible reaction occurs; Gas is produced and some precipitate dissolves
Step-by-step explanation: When aqueous solutions of
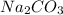 and
and
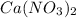 are combined, it reacts according to the following balanced equation:
are combined, it reacts according to the following balanced equation:
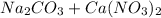 →
→
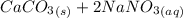
forming calcium carbonate (
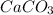 ), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction.
), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction.
When calcium carbonate reacts with NaCl it produces:
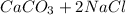 →
→
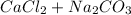
Now, calcium chloride is an inorganic compound very soluble in water, so, in this reaction, there are no precipitate and no visible reaction occurs.
When
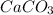 reacts with hydrochloridric acid, the balanced reaction is
reacts with hydrochloridric acid, the balanced reaction is
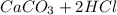 →
→
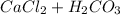
which, also produces calcium chloride and carbonic acid.
Both are soluble in water but, when carbonic acid is in an "aqueous state", carbonic acid, it dissociates, forming carbon dioxide and water. Therefore, gas is produced and some precipitate dissolves.
In conclusion, sentence B is the correct alternative.