Answer: The amount of heat released is -1046 J
Step-by-step explanation:
To calculate the amount of heat released, we use the equation:
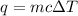
where,
q = heat released = ?
m = mass of water = 25.0 g
c = specific heat capacity of water = 4.184 J/g.°C
 = change in temperature =
= change in temperature =
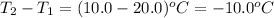
Putting values in above equation, we get:
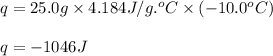
Hence, the amount of heat released is -1046 J