Answer:
B) The gas that was released changed the mass.
Step-by-step explanation:
In the acid base reaction of vinegar and sodium bicarbonate, sodium acetate, water and carbon dioxide are formed.
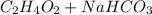 →
→
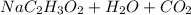 ↑
↑
Carbon dioxide is released from the beaker in the form of gas (as can be seen in the previous reaction), which is due to the loss of mass observed. This compound is responsible for the formation of bubbles in the reaction.