Answer: The correct answer is Option a.
Step-by-step explanation:
Combustion reaction is defined as the reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule. It is considered as an exothermic reaction as energy is released during these reactions.
A hydrocarbon is a substance which contains a covalent bond between a hydrogen and carbon atoms. So, it is considered as a carbon-fuel compound.
The general equation for this reaction follows:
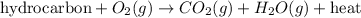
From the above equation, it is visible that elemental carbon is not produced.
Hence, the correct answer is Option a.