Answer:
- 0.75 liters of 5% hydrochloric acid solution
- 0.25 liters of 45% hydrochloric acid solution.
Explanation:
Let us assume that, x liters of the 5% hydrochloric acid and y liters of the 45% hydrochloric acid solutions are combined.
As Rajan need total of 1 liter of solution, so
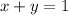
i.e
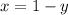 --------------------1
--------------------1
As Rajan needs 5% hydrochloric acid and 45% hydrochloric acid to make a 1 liter batch of 15% hydrochloric acid, hence acid content of the mixture of two acids will be same as of the final one, so
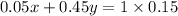
i.e
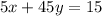 -------------2
-------------2
Putting value of x from equation 1 in equation 2,
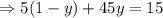
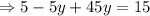
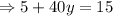
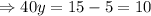
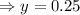
Putting the value of y in equation 1,
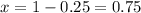
Therefore, Rajan must use 0.75 liters of 5% hydrochloric acid solution and 0.25 liters of 45% hydrochloric acid solution.