Neutron is a subatomic particle found in the nucleus of an atom except in the nucleus of hydrogen. Since it does not have any charge and it is a neutral atom it is known as neutron. Neutron has a symbol n or
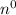 . The mass of single atom is
. The mass of single atom is
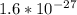 ,. Proton is also a subatomic particle found in the nucleus of an atom with the symbol p or
,. Proton is also a subatomic particle found in the nucleus of an atom with the symbol p or
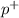 . Proton carries positive charge. Electron is the subatomic particle which revolves round the nucleus and it carries negative charge.
. Proton carries positive charge. Electron is the subatomic particle which revolves round the nucleus and it carries negative charge.
Therefore the answer is X is neutron since it is an uncharged particle Y is a proton since it carries positive charge and Z is an electron since it revolves round the nucleus or orbits the nucleus.