Answer: The average atomic mass of the lithium ion remains the same as lithium atom which is 6.94 amu
Step-by-step explanation:
Mass number is defined as the sum of number of protons and number of neutrons present in an atom. It is represented as A.
A = Mass number = Number of neutrons + Number of protons.
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
So, the loss or gain of electrons does not effect the mass number of an atom. Thus, when lithium atom looses an electron to form lithium ion
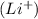 , the average atomic mass of the ion remains the same as the neutral atom.
, the average atomic mass of the ion remains the same as the neutral atom.
Average atomic mass of lithium atom = 6.94 amu
Hence, the average atomic mass of lithium ion is 6.94 amu.