Answer:
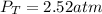
Step-by-step explanation:
Hello!
In this case, since we are analyzing the pressure-volume behavior of the gas, we need to use the Boyle's law as an inversely proportional relationship:
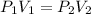
Thus, the final pressure in each bulb, once the gases have mixed, resulting in a volume of 5.00 L, is:
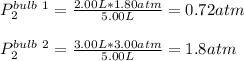
It means that the final total pressure, via the Dalton's law is:
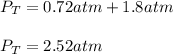
Best regards!