Answer:
The density of gas particles inside tires is higher than the density of the gas particles outside.
Step-by-step explanation:
Let suppose that both gas inside and outside tires behave ideally. The equation of state for ideal gases is presented below:
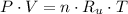 (1)
(1)
Where:
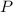 - Pressure.
- Pressure.
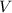 - Volume.
- Volume.
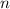 - Molar quantity.
- Molar quantity.
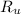 - Ideal gas constant.
- Ideal gas constant.
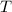 - Temperature.
- Temperature.
By definition of molar quantity, we expand (1) into this form:
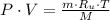
And after some algebraic handling, we derive the following formula for the density of the gas:
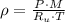 (2)
(2)
The gas inside tires has a pressure higher than the pressure outside, but the same temperature usually. Therefore, the density of gas particles inside tires is higher than the density of the gas particles outside.