Answer: The number to the left of AC should be 6.
Explanation: The balanced chemical reaction is one in which the number of atoms of each element on the reactant side must be equal to the number of atoms on product side.
The given equation
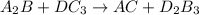 is unbalanced as the atoms on the reactant side are not same as number of atoms on product side. This equation is called as skeletal equation.
is unbalanced as the atoms on the reactant side are not same as number of atoms on product side. This equation is called as skeletal equation.
The balanced chemical equation is :
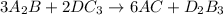
Thus the number to the left of AC is 6.