Answer:
The mass defect of a deuterium nucleus is 0.001848 amu.
Step-by-step explanation:
The deuterium is:
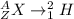
The mass defect can be calculated by using the following equation:
![\Delta m = [Zm_(p) + (A - Z)m_(n)] - m_(a)](https://img.qammunity.org/2022/formulas/chemistry/college/dal87k8anckkw4vz24r2p1gtmv9ux2rkr6.png)
Where:
Z: is the number of protons = 1
A: is the mass number = 2
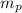 : is the proton's mass = 1.00728 amu
: is the proton's mass = 1.00728 amu
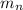 : is the neutron's mass = 1.00867 amu
: is the neutron's mass = 1.00867 amu
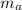 : is the mass of deuterium = 2.01410178 amu
: is the mass of deuterium = 2.01410178 amu
Then, the mass defect is:
![\Delta m = [1.00728 amu + (2- 1)1.00867 amu] - 2.01410178 amu = 0.001848 amu](https://img.qammunity.org/2022/formulas/chemistry/college/mua00fzu0ty381v6qaqhmob8ehewzkfm35.png)
Therefore, the mass defect of a deuterium nucleus is 0.001848 amu.
I hope it helps you!