Answer: 8184.96 J
The ice would reach a temperature 0° C. It would melt to form water and again gain heat to reach ambient room temperature of 20° C.
Therefore, the heat would be absorbed for the three processes,
The rise of temperature from -18°C to 0°C, Q₁= m c ΔT
where, c is specific heat of ice
Q₁= m c ΔT = 18 g × 2.00 J/g° C × 18° C = 648 J
Melting of ice at 0°C, Q₂ = m L = 1 mol × 6030 J/mol = 6030 J
where L is the latent heat of fusion
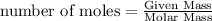
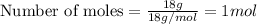
The rise of temperature of water from 0°C to 20°C, Q₃= m c' ΔT'
Where, c' is the specific heat of water.
Q₃= 18 g × 4.186 J × 20° C = 1506.96 J
Net heat absorbed:
Q = Q₁ + Q₂ + Q₃ = 648 J+6030 J+1506.96 J = 8184.96 J
Hence, 8184.96 J heat must be absorbed to make this happen.