In a precipitation reaction, one of the product formed is an insoluble precipitate.
The given reactions can be written with phases included as:
A.
 : In this reaction one of the product, FeS is insoluble. Therefore, this is a precipitation reaction.
: In this reaction one of the product, FeS is insoluble. Therefore, this is a precipitation reaction.
B.
 : In this reaction, the product
: In this reaction, the product
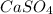 is a solid(insoluble). So, this is a precipitation reaction too.
is a solid(insoluble). So, this is a precipitation reaction too.
C.
 : In this reaction, both the products are soluble. So this is not a precipitation reaction.
: In this reaction, both the products are soluble. So this is not a precipitation reaction.
D.
 : In this reaction, both the products are soluble. So this is not a precipitation reaction.
: In this reaction, both the products are soluble. So this is not a precipitation reaction.
E.
 : In this reaction, the product AgCl is a precipitate. So, it is a precipitation reaction.
: In this reaction, the product AgCl is a precipitate. So, it is a precipitation reaction.