Answer:
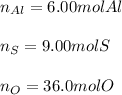
Step-by-step explanation:
Hello!
In this case, when analyzing the moles of an element inside a compound, we need to keep in mind that a mole ratio should be set up; thus, for aluminum sulfate, we have the following ones:

Thus, starting by 3.00 moles of aluminum sulfate, the moles of each element turn out:
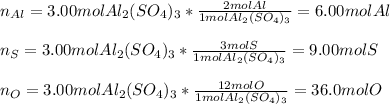
Best regards!