Answer:
As has more ionization energy.
Step-by-step explanation:
Ga is the element of forth period and 13 group.
The electronic configuration is -
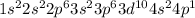
As is the element of forth period and 15 group.
The electronic configuration is -
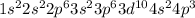
Since, As is half filled in 4p orbital it is more stable. Hence, more energy is required to knock out the valence electron.
Hence, As has more ionization energy.