Answer:
36.8 g of oxygen would form from 2.30 moles of water
Step-by-step explanation:
According to given balanced equation, 2 moles of water forms 1 mole of oxygen
So moles of oxygen would form from 2.30 moles of water=
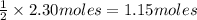
Molar mass of
 is 32 g/mol
is 32 g/mol
So mass of
 would form=
would form=
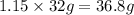
hence 36.8 g of oxygen would form from 2.30 moles of water