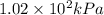Answer : The absolute pressure of the air in the balloon is
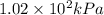
Explanation :
Using ideal gas equation :
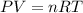
where,
P = Pressure of the air in the balloon = ?
V = Volume of the balloon = 6.23 L
n = number of moles of air filled in balloon = 0.250 mole
R = Gas constant =
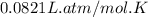
T = Temperature of the balloon =
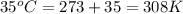
Putting values in above equation, we get:
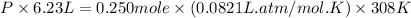
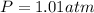
Now we have to convert the pressure of the air from 'atm' to 'kilopascals'.
Conversion used :
1 atm = 101.325 kPa
So, 1.01 atm = 1.01 × 101.325 = 102.34 kPa ≈
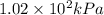
Therefore, the absolute pressure of the air in the balloon is