Answer:
The number of moles of calcium atom in each mole of calcium carbonate is 1 mole of calcium atoms
Step-by-step explanation:
The chemical formula for CaCO₃ = 100.086 g
The number of molecules in one mole of a substance is given by the Avogadro's number,
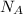 = 6.022 × 10²³ molecules
= 6.022 × 10²³ molecules
The number of calcium atoms in one molecule of CaCO₃ = 1 atom of calcium
Therefore, the number of calcium atoms in 1 mole of CaCO₃ = 1 × 6.022 × 10²³ atoms of calcium = 6.022 × 10²³ atoms of calcium
6.022 × 10²³ atoms of calcium = The number of toms in 1 mole of calcium atoms
Therefore, the number of moles of calcium atom in each mole of calcium carbonate = 1 mole of calcium atoms.