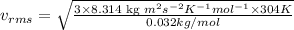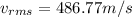Answer: The rms speed of an oxygen gas molecule is 486.77 m/s.
Explanation: The expression for the rms speed of the molecule is given by:
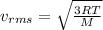
where,
R = Gas constant = 8.314 J/K mol =
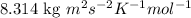
T = Temperature of the gas = 31°C = (273 + 31)K = 304K
M = Molar mass of the gas =
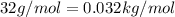 (Gas is Oxygen)
(Gas is Oxygen)
Putting values in above equation, we get: